
Menu
Our Blog
October 2, 2023
The CDISC Standards: The Success Formula for Clinical Data
In the world of clinical research, data exchange and analysis play a key role in the development of safe and effective treatments. The Clinical Data Interchange Standards Consortium (CDISC) have proven to be the formula for success in achieving effective harmonization and standardization in clinical data exchange. In this article, we will explore how CDISC standards have revolutionized the way data is presented, analyzed and shared in medical research, improving the quality of clinical trials and accelerating progress towards new therapeutic advances.
By Mercedes Ovejero and Jaime Ballesteros
Biostatistics Unit of Sermes CRO Sermes CRO
Introduction to CDISC
In the field of clinical research and the development of new medical treatments, data collection, management and analysis play a key role. However, for many years, clinical data have been presented in a wide variety of formats that have not followed common organizational and structural criteria. This situation has hindered the efficient exchange of information between investigators, regulators and the pharmaceutical industry, making it necessary to implement a strategy to share and compare clinical trial data under internationally recognized standards.
To address this issue, the Clinical Data Interchange Standards Consortium (CDISC), a global non-profit organization that has become a leader in the development of standards for the exchange of clinical data, was created. Since its founding, CDISC has worked tirelessly to establish standards that improve the quality, efficiency and consistency of clinical data management in clinical trials and research studies.
The CDISC Objectives: Improving the Efficiency and Quality of Clinical Research
The main objective of CDISC is to develop and promote standards that facilitate the collection, management and analysis of clinical data throughout the life cycle of a study. By adopting these standards, clinical data can be presented in a structured and standardized way, streamlining the analysis, reporting and regulatory review processes.
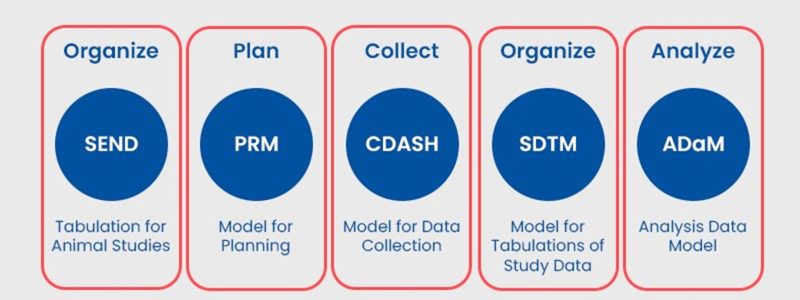
CDISC covers various aspects of the clinical research process, from data collection to reporting, and proposes the use of the following models to carry out its classifications:
- SEND (Standard for Exchange of Nonclinical Data): it is a standard for the submission of non-clinical data in pre-clinical studies. SEND establishes a standardized structure and terminology for organizing and submitting non-clinical data, which facilitates communication and information exchange between the pharmaceutical industry and regulatory agencies.
- PRIM (Protocol Representation Model): this standard provides a model for the representation and description of the elements of a clinical research protocol. PRIM allows for the structured capture of information related to study design, inclusion and exclusion criteria, study visits and expected outcomes, which promotes consistency and transparency in clinical trial design.
- CDASH (Clinical Data Acquisition Standards Harmonization): it focuses on the standardization of clinical data acquisition during the collection process. CDASH proposes a set of codes and associated metadata that enable the processing of information collected directly at the site level, facilitating the consistent and efficient capture of clinical data in clinical studies.
- SDTM (Study Data Tabulation Model): it defines a standardized structure for organizing and presenting tabular data from clinical studies, operationalizing the variables collected in the databases according to pre-established categories, allowing the efficient presentation and exchange of data between different systems and organizations.
- ADaM (Analysis Data Model): it is a standard that focuses on the structure and organization of analysis data derived from clinical studies. This model establishes a consistent and standardized structure for data that is to be used in statistical analyses and generation of key results in clinical research. ADaM allows data tabulated in SDTM to be converted into analytical datasets, ready to apply statistical techniques and answer the research questions posed in the study protocol. By providing a clear and consistent structure for analytical data, ADaM improves the efficiency of the knowledge generation and decision-making process, providing accurate and reliable results that support the safety and efficacy of treatments and medicinal products developed in clinical research.
Benefits of the Adoption of CDISC Standards
The adoption of CDISC standards brings multiple benefits to the clinical research community and the pharmaceutical industry. Among the main ones are:
- Regulatory Compliance: The adoption of CDISC standards helps pharmaceutical companies to comply with the requirements of regulatory agencies, streamlining the approval process for new treatments and medicinal products.
- Reproducibility: By using a standard structure for data reporting, studies can be more easily reproduced by other investigators, increasing transparency and confidence in the results.
- Efficiency: Standardization of data and processes reduces duplication of effort and time spent on data preparation, which improves the overall efficiency of clinical research.
- Data Quality: CDISC standards promote the consistency and quality of data collected in clinical studies by standardizing the structure and definition of variables. This reduces the possibility of errors and facilitates greater reliability in the results obtained.
- Facilitates Data Integration: CDISC standards allow for easier integration of data from different sources and clinical studies. By using a standardized structure, data can be combined and analyzed together, providing a more complete and holistic view of information. This data integration can provide deeper and more insightful knowledge about the effects of treatments and the evolution of diseases, thus enriching medical research and clinical decision-making.
- Facilitates Analysis and Decision Making: The use of CDISC standards streamlines the data analysis process by having a consistent and well-defined structure. This enables faster and more effective interpretation of results, facilitating informed decision-making in the development of medicinal products and therapies.
- Global Collaboration: The adoption of CDISC standards promotes global collaboration in clinical research. By having a common structure for data reporting, investigators from different countries and organizations can work more smoothly and collaboratively on multi-center projects.
- Traceability and Auditability: CDISC standards allow for better traceability and auditability of data, which facilitates the review and verification of the integrity of the results obtained. This is especially relevant in the regulatory context, where accurate and transparent documentation of submitted data is required.
In short, CDISC has revolutionized the way clinical data is managed and presented, setting standards that have transformed the pharmaceutical industry and medical research. Its focus on standardization and collaboration has driven positive change in the quality and efficiency of clinical research, paving the way for a promising future in fighting disease and improving global healthcare.
Follow us to learn more about each of the CDISC standards and their importance in clinical research.
Certifications:














